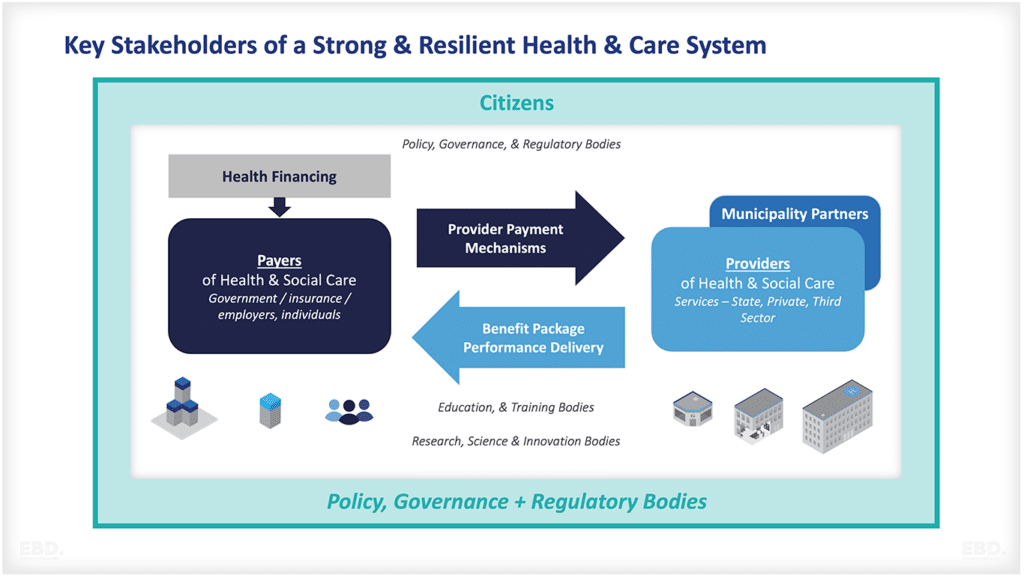
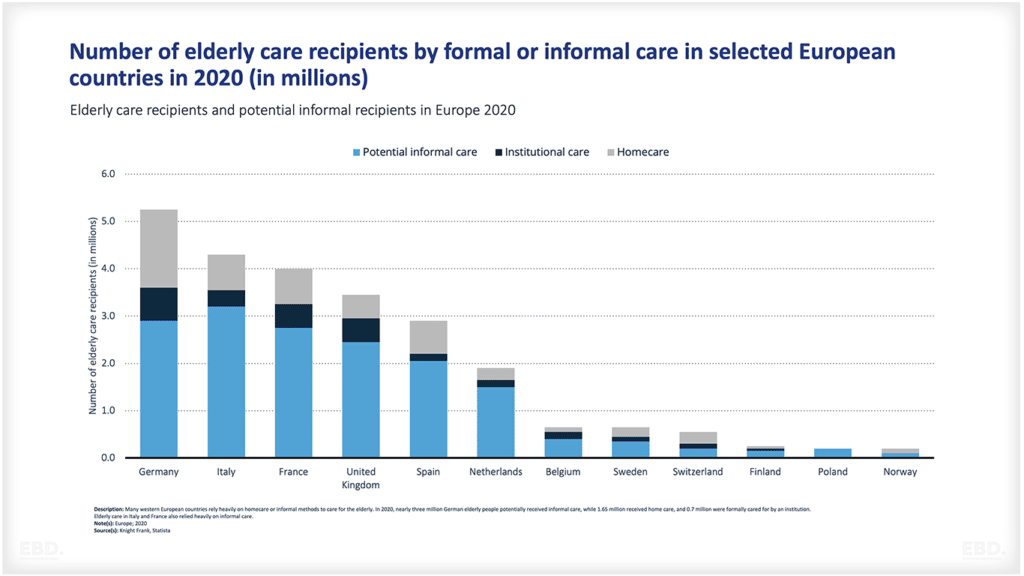
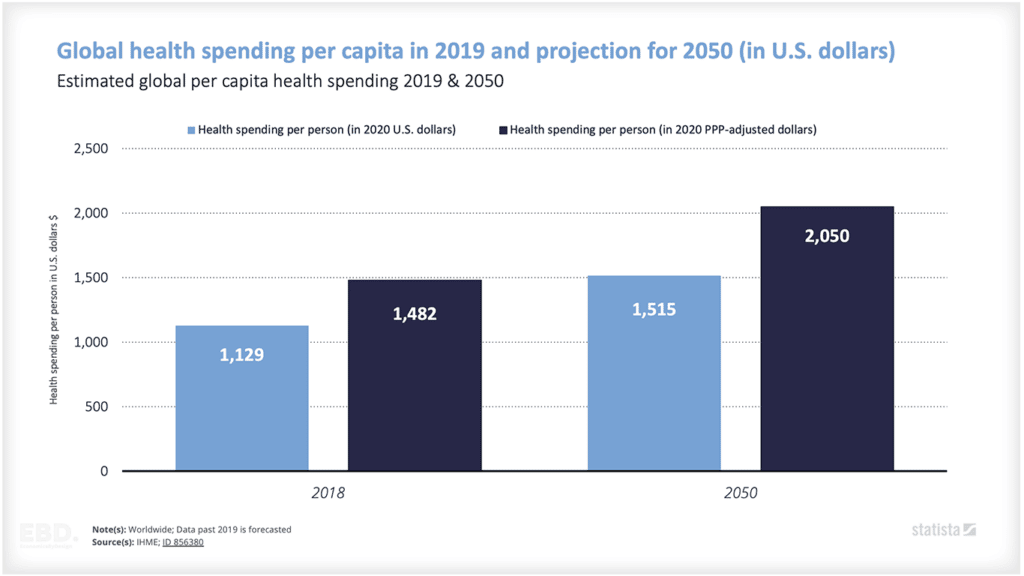
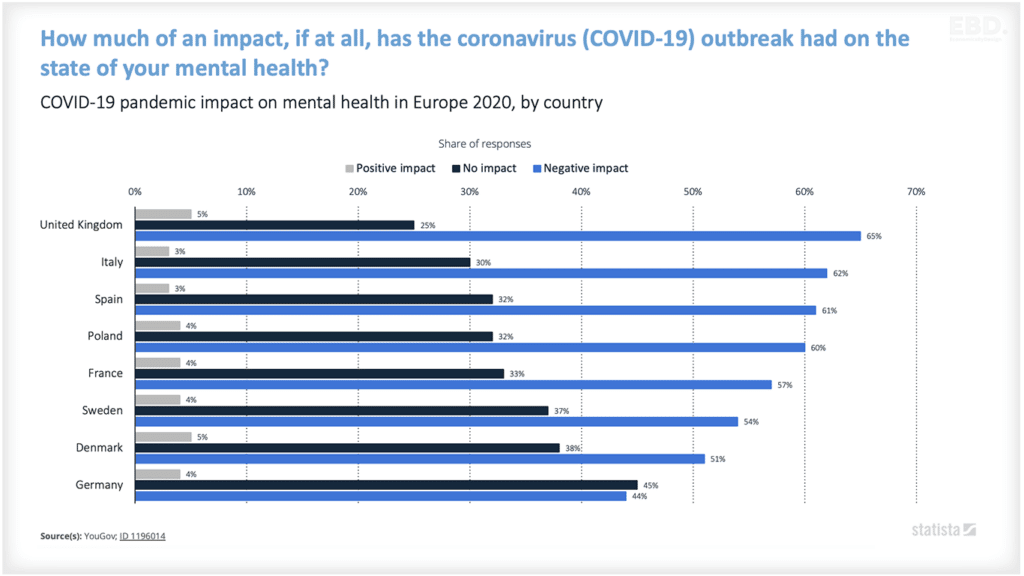
Why is there Research And Development in Healthcare?
Health research and development is really important to the health sector. It provides clinical practitioners and health system investors with innovation and associated evidence for prevention interventions, effective treatments and care pathways.
In this economic lens we provide an overview of health research and development and its role in the health system.
What is Health Research And Development?
Research and development is fundamentally about identifying a need or a problem, generating an idea or solution, converting that idea into a product or service, testing the product or service for efficacy, effectiveness and value, launching the product or service, continuing to monitor, evaluate and improve the product or service, and then finally sunsetting the product or service when it becomes obsolete.
Typically, products include medical devices, drugs and vaccines, gene therapy solutions, diagnostic tools. They can also include services and care-pathways including prevention, treatment and care, and rehabilitation.
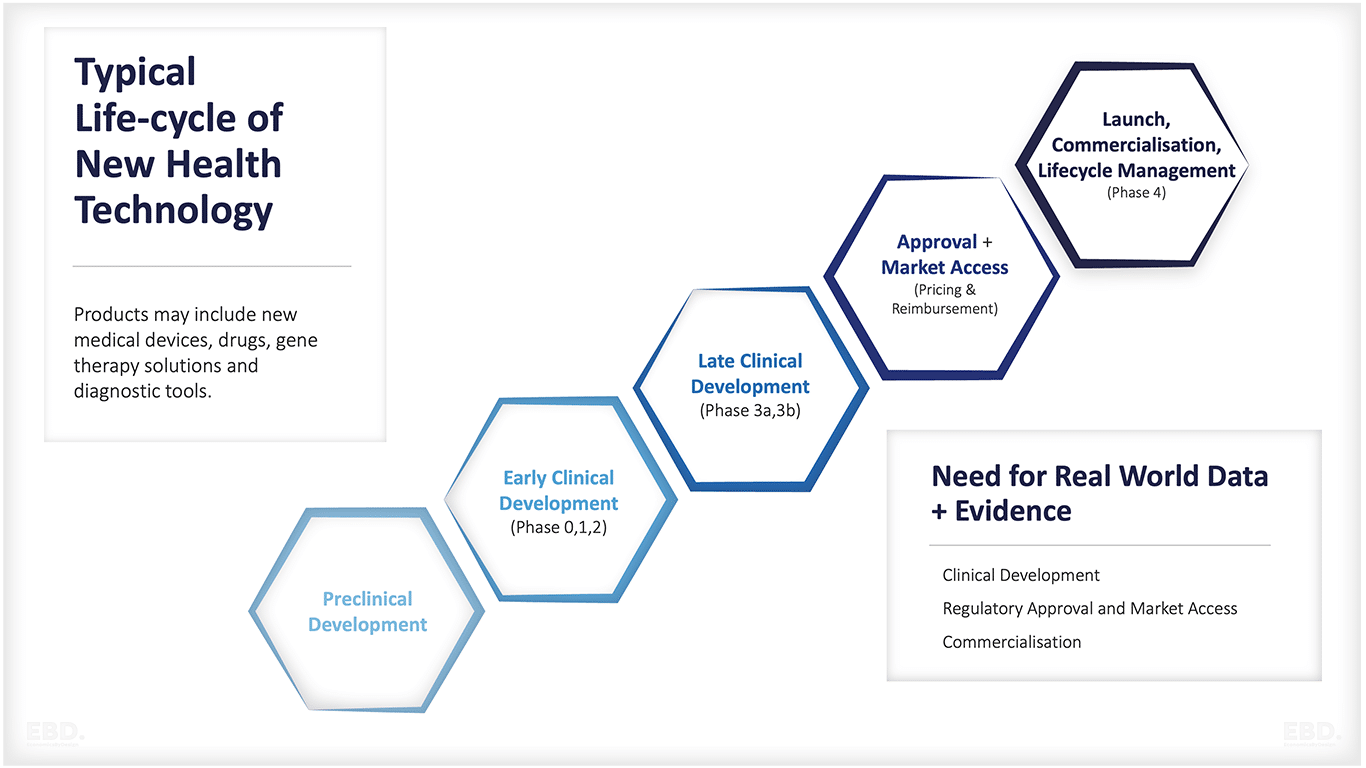
The key stages of health research and development are:
Pre-clinical development
This covers all activities that happen before a product or service is tested on people. For clinical interventions such as drugs, medical devices, gene therapy, diagnostic tools etc, this will include feasibility testing, safety testing etc. often in laboratory settings.
Some pre-clinical development is undertaken using animals. In vitro testing is done “outside the body” or “outside the normal biological context” for example in a test tube or a petri-dish. In vivo is when testing is done “within the living” and, in the case of pre-clinical development, this is animal testing.
Often pre-clinical development can include what are known as “observational studies”. This is when the health status of a population or population subgroup is monitored to identify where problems exist, and where prevention and treatment interventions might work.
Early clinical development
This is when testing is done with people. Usually, at these early stages, the drug or device is tested for safety or dosage and to see how the clinical intervention impacts humans. This stage often involves, “stages 0, 1 and 2” clinical trials which are research studies which test the impact of a product or service on people. The stage of the trial generally reflects the number of participants which increases as more is learned about dosage, safety, and side-effects.
Late clinical development
This is the stage where the product or service is tested at scale on a population. These stage 3 trials are complex, time-consuming, and often expensive. The trial aims to test the impact of the product or service on health outcomes.
It is important to isolate the impact of the product or service from other factors which independently explain changes in health outcomes. For these reasons, stage 3 clinical trials are often designed as randomised controlled trials (RCTs).
An RCT involves randomly allocating trial participants into different groups, those receiving the intervention (the treatment group) and those who are not (the control group). Often this is done “Blind” so that the participant doesn’t actually know whether they are receiving the intervention or not. In the case of a drug, they may, for example, receive a “placebo” (a treatment with no therapeutic value).
Sometimes, the stage 3 trial can be “double-blind” where the health practitioner delivering the intervention does not know which participant is receiving the treatment or the placebo.
If the trial population is representative of the general population or the target population for the treatment, then the RCT process can ensure that all factors that might influence the health outcome (such as age, other health conditions, fitness etc.) are equally likely to interfere with the treatment group and the control group and can therefore be ignored. The only reason for a different health outcome between the treatment group and the control group is the therapeutic value of the product or service.
In practice, it is very hard and/or expensive to design RCTs and so often, stage 3 trials use other techniques to minimise the risk of what is known as “attribution bias”, namely that observed differences in outcomes for the treatment group is mistakenly attributed to the product or service.
Importantly, if the product or service requires regulatory approval, stage 3 clinical trials are usually run after an application for regulatory approval is filed, but before it is awarded.
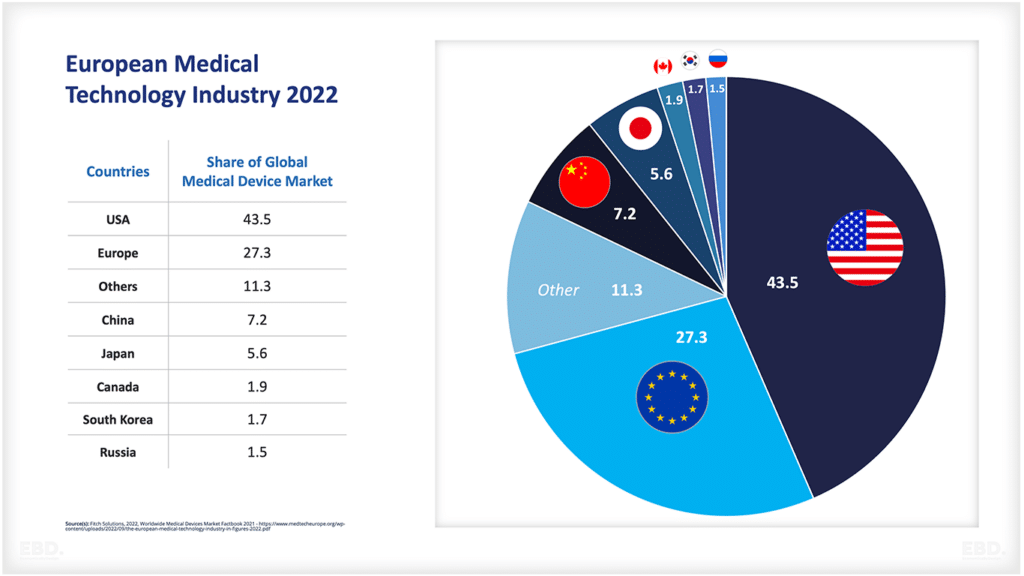
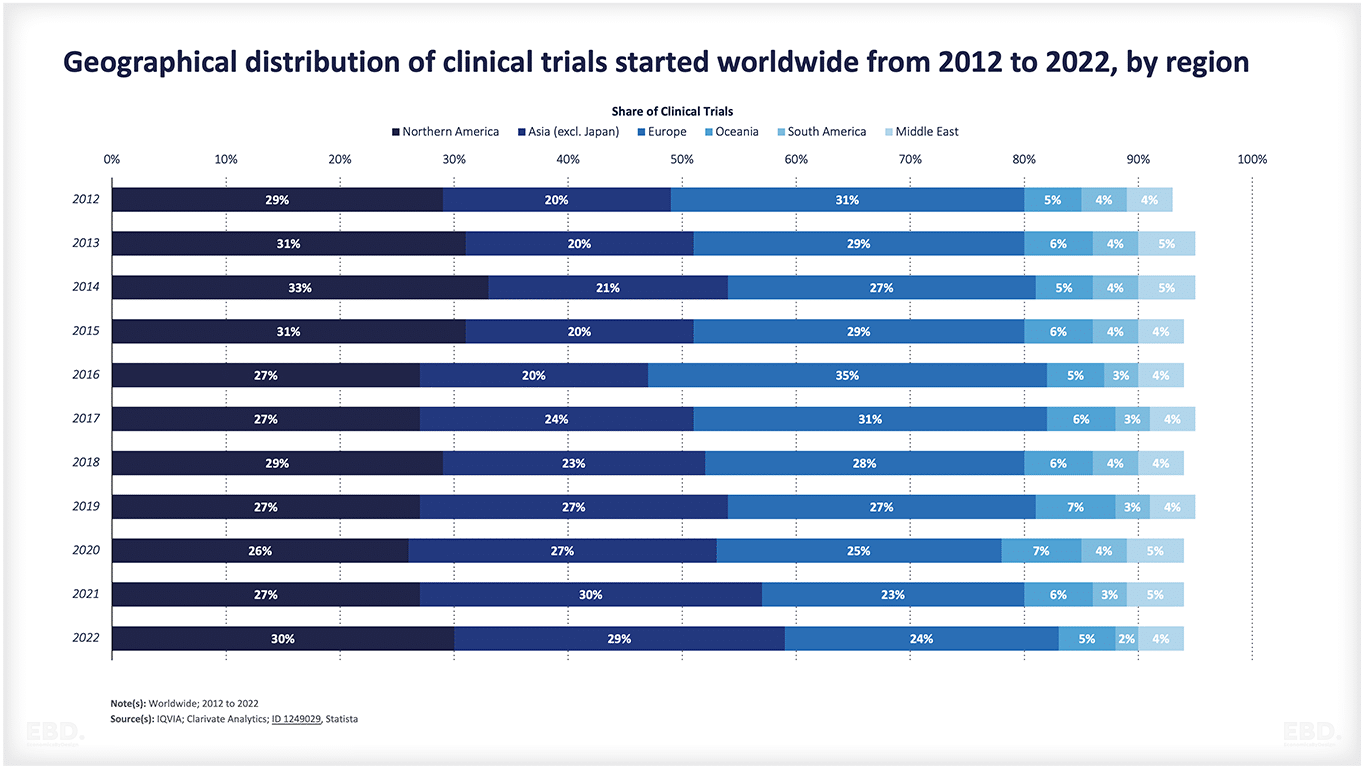
The chart below shows that, at present, the vast majority of clinical trials are undertaken in North America, Asia and Europe.
Market Authorisation, Pricing and Reimbursement
This stage is where the product or service is approved by the relevant regulatory body (if required) and is when pricing and reimbursement decisions are made with those responsible for paying for or commissioning healthcare on behalf of a population.
This stage often requires additional information to be collected, and in particular economic data to demonstrate the value of the product or service. This is often known as Health Technology Appraisal (HTA). The organisation that has developed the product or service will be asked to submit data to support this process and the payer will commission independent experts to undertake the HTA.
For some payers, there are clear benchmarks or guidelines relating to effectiveness and value which need to be achieved if the product or service is to be included in the health benefits package. For others, decisions are taken on a case-by-case basis. Often decisions to include products or services as part of essential health benefits are taken on the advice of a specialist committee comprising experts in the field as well as patient representatives.
Increasingly, payers and regulators are linking approval, pricing and reimbursement to a requirement that the product or service undergo some additional data collection and surveillance requirements to assure effectiveness and value.
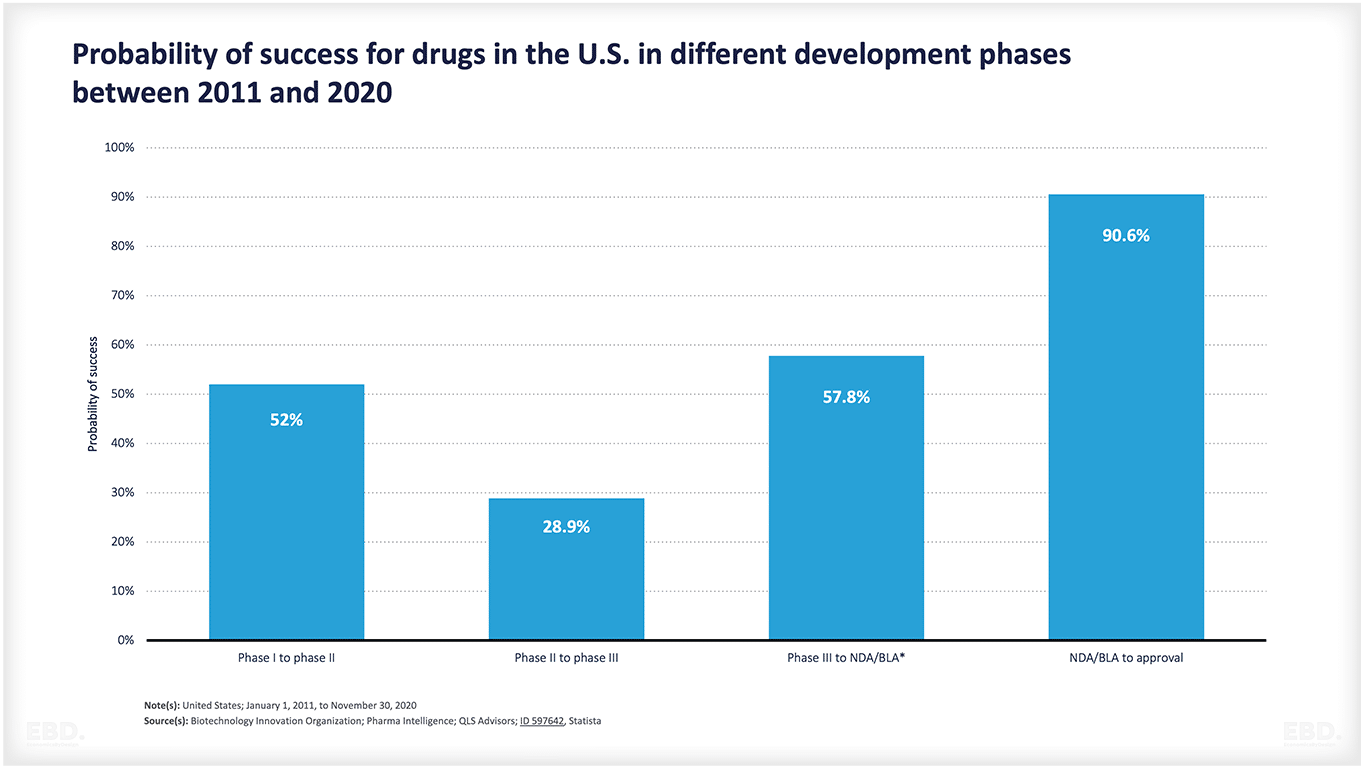
As the chart below indicates, many products do not make it through from conception to approval. There is a lot of scientific or technological uncertainty involved. This chart shows that for drugs in the USA, around half move from stage 1 to stage 2 and around 29% of these move from stage 2 to stage 3. Over half then move on to the market access stage and, if they get there over 90% get approved. Collectively though, only 7.8% of drugs move from pre-clinical to approval. That is a huge amount of resources devoted to products that do not make it to commercial launch.
Launch, Commercialisation, Life Cycle Management
This phase covers all research and development activities relating to a health product or service once it is has been adopted for deployment across a population. Activities here include monitoring for safety and side effects, monitoring for impact, and also gathering real-world evidence to inform decisions as to whether the product might expand its population coverage or the types of health conditions it can help with.
What Is Real World Data And Evidence?
Increasingly, the research and development stakeholders in health systems have been looking to the use of real-world data and evidence to inform decisions. Real-world data are data collected routinely from within the health system and beyond. Real-world evidence is based on the analysis of real world data.
Potential uses for real-world data and evidence include:
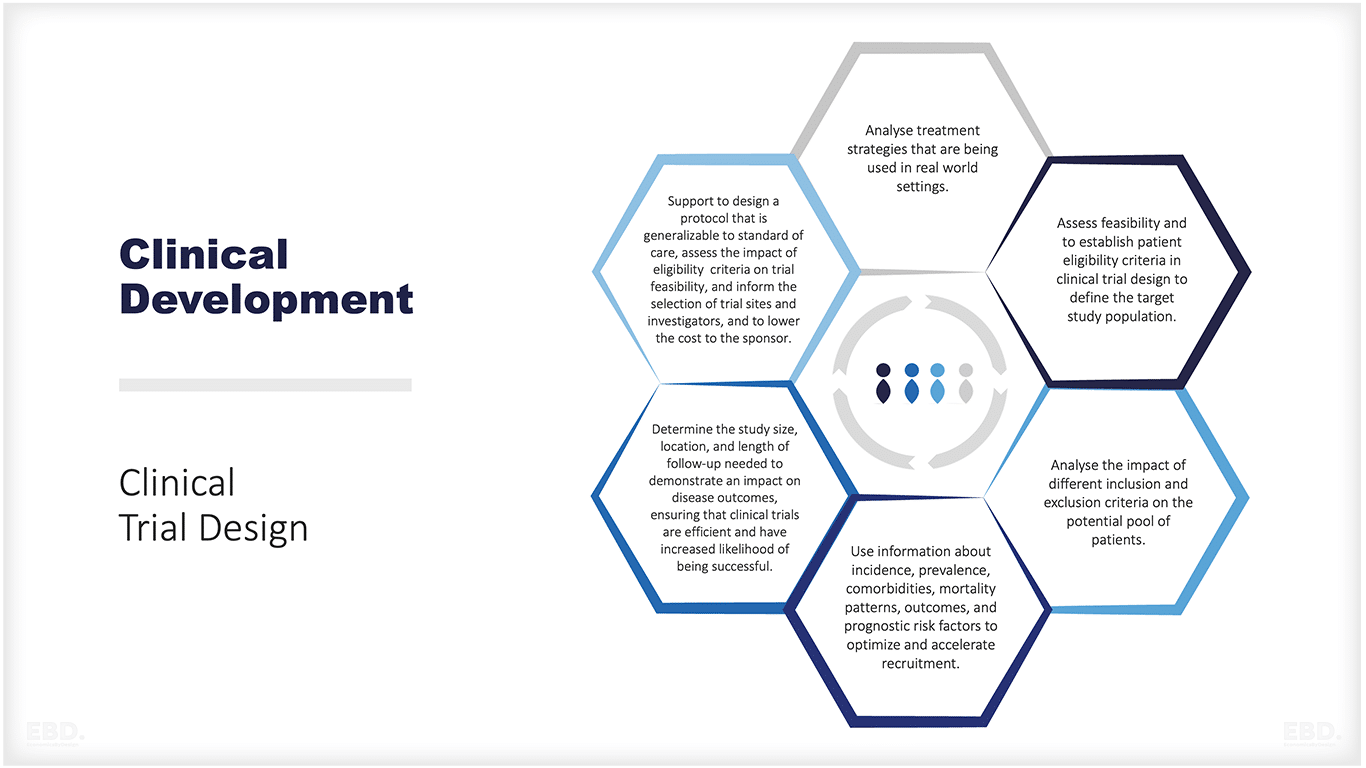
Clinical Development
- Prioritise early clinical development
- Study the disease and its burden
- To study the disease Management
- Clinical Trial Design
- Trial Execution Increase patient recruitment efficiency
Regulatory Approval + Market Access
- Facilitate Regulatory Process
- Facilitate Pricing, Reimbursement and Market Access
Commercialisation
- Provide new scientific and clinical insights
- Provide commercial and Business Insights
- Support commercial programmes
- Facilitate post-marketing pharmacovigilance and surveillance
- Patient Management Optimization
- Indication expansion
The figure below provides a summary of the types of issues where real-world data can support the design and implementation of an effective clinical trial.
Characteristics of an ideal data source to generate real-world evidence
- Patient population representative of those with underlying condition (e.g., demographics, clinical comorbidities)
- Prospectively Planned
- Continuously updated with minimal resources
- Longitudinal follow-up
- Rich clinical date: clinician entered, patient reported and patient generated
- Quality control measures in place
- Integrated within existing data systems
There are challenges with real-world data. These data can be of variable quality in terms of accuracy, they may also be partial in coverage. Real-world data are not necessarily regular or timely, and may not provide comprehensive information across all areas of interest. However, as the digital capabilities of health systems mature, these issues are becoming less of an obstacle to the use of real-world evidence by the research and development community.
What Is Research Governance?
Research and development in healthcare present many ethical issues. Research participants are being asked to be human test beds for new products and services that may not work, or worse, may harm. They are often asked to participate because they are living with a condition or disease already. They are also being asked to provide very personal data and information to a research team.
Research governance is defined as the regulations, rules, principles and standards that ensure and assure high-quality research. Well-designed research governance should provide assurance to all stakeholders that the research is being done to the required quality standards, has a purpose and value, and that standards for participation, protection of participants and researchers, and sharing of information are being adhered to.
It should also enable monitoring of performance and sharing of good practices in healthcare, research governance covers all health-related research which involves people, their tissue or their data.
Arrangements for research governance vary across the world. In some countries, there are strong arrangements in place and approval is required for all research involving people, their tissue or their data. Ethics approval will be conditional on the quality of the research protocol, and consent arrangements being in place for participants.
In some countries, these arrangements are not yet established. Where the research is undertaken in countries without research governance in place and/or where ethical approval was not given, it can be difficult for researchers to see their research published in peer-reviewed journals.
Useful examples of research governance arrangements and organisations include:
- The European Clinical Trials Regulation which governs clinical trials across the European Commission
- The NHS Health Research Authority provides a policy framework for good practice in the management and conduct of health and social care research in the UK
- The USA National Institute of Allergy and Infectious Diseases (NIAD)’s ClinRegs website provides country-specific information on clinical research regulations in selected countries globally.
How Much Investment Is There In Health Research And Development?
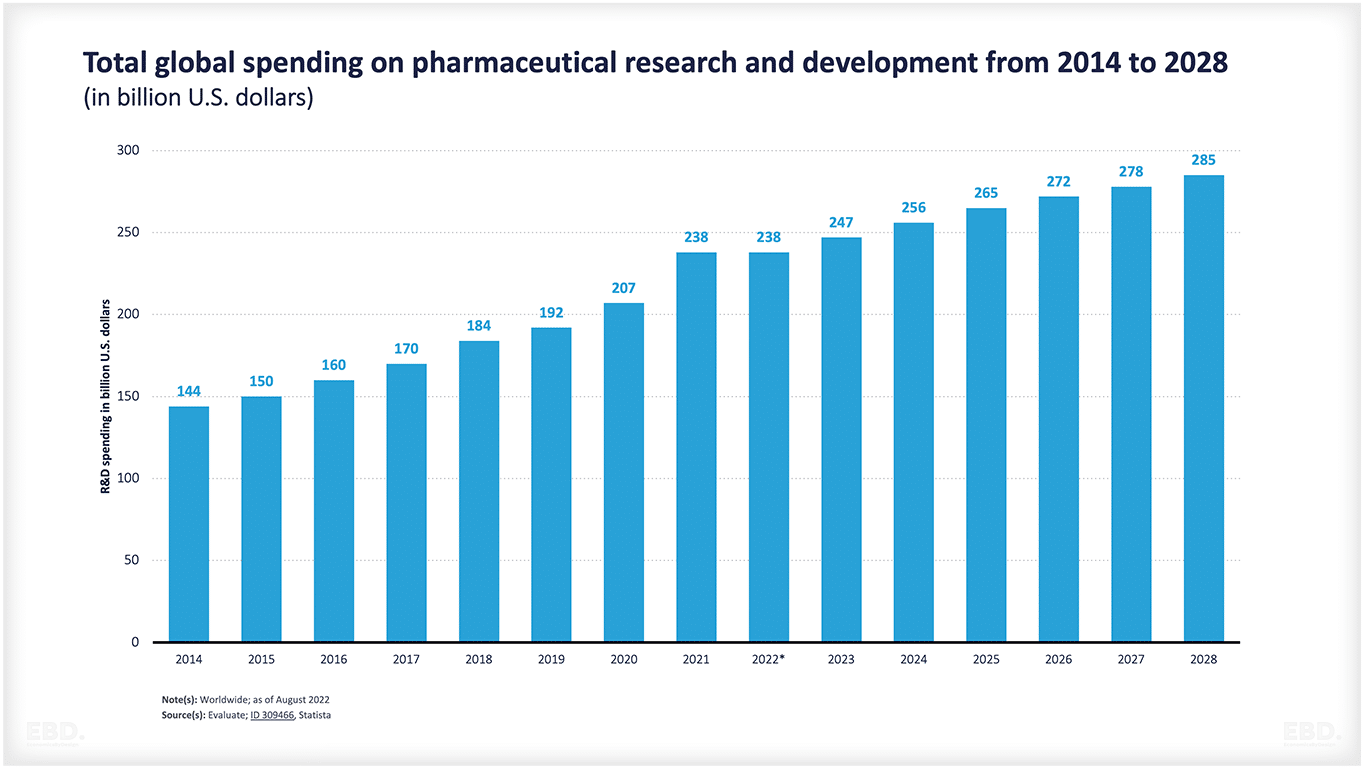
According to the UNESCO Institute of Statistics , the world spent the equivalent of $2.47tn on research and development in 2022 Of this, $238bn relates to pharmaceutical research and development and this is expected to grow to $285bn by 2028.
There are many stakeholders involved in funding research and development including private companies (e.g. pharmaceuticals, medical device companies, digital health companies), government through the provision of grants and awards, and through hosting research in government-run health service providers, and medical research charities.
Examples of top global pharmaceutical companies that invest in research and development include:
Top philanthropic funders of health research globally include:
- Wellcome Trust
- Howard Hughes Medical Institute
- Bill and Melinda Gates Foundation
- Insitute Pasteur
- Oswaldo Cruz Foundation
- Rockefeller Foundation
Who Delivers Health Research And Development?
Most health research and development is delivered by clinical academics, clinicians funded by research funders, and academic healthcare providers who host research.
Academic Health Science Centres are health care providers who combine research, education and service delivery. Examples include:
- Johns Hopkins (School of Public Health, School of Nursing, School of Medicine) in the USA
- UCL Partners in England
- Npistanbul Brain Hospital in Turkey
- Leiden University Medical Centre in Netherlands
What Is The Value Of Government-Funded Health Research & Development?
Governments fund health research and development to stimulate investment from the philanthropic and commercial sectors, and to provide access to funds in areas where commercial incentives are relatively poor.
Government-funded health research and development can therefore add value, both by stimulating economic growth and by supporting health improvement for the population and individuals.
There is very little research available to show the return on investment from government funding for research and development, but some studies have shown positive returns for selected case studies.
- Grant J, Buxton MJ Economic returns to medical research funding BMJ Open 2018;8:e022131. doi: 10.1136/bmjopen-2018-022131 showed returns of between 7% and 10% in terms of health gain, and a further 15% from the wider economic impact. This was based on investment in cardiovascular, cancer and musculoskeletal research in the UK.
- Joyce Craig and colleagues at the University of York estimated a return on investment from the funding of Biomedical Research Centres in the UK of around 29%.
- A recent study from a consortium of Kings College London, RAND Europe, and Brunel University suggested a return on investment of around 25% (based on musculoskeletal disease research in the UK).
The most important factor to consider when looking at the investment value of research and development in healthcare is that it is inherently uncertain. This is where health systems need to be prepared to take risks and/or support companies that are prepared to take risks.
Global Observatory on Health R&D
One of the best sources of information on global health research and development is the World Health Organisation Global Observatory on Health R&D. It provides information and analysis based on a synthesis of data and information from across the world on research and development of disease.
It is a truly rich resource for anyone wanting to find out what is being researched and where gaps exist. It is a one-stop shop for researchers in the field.