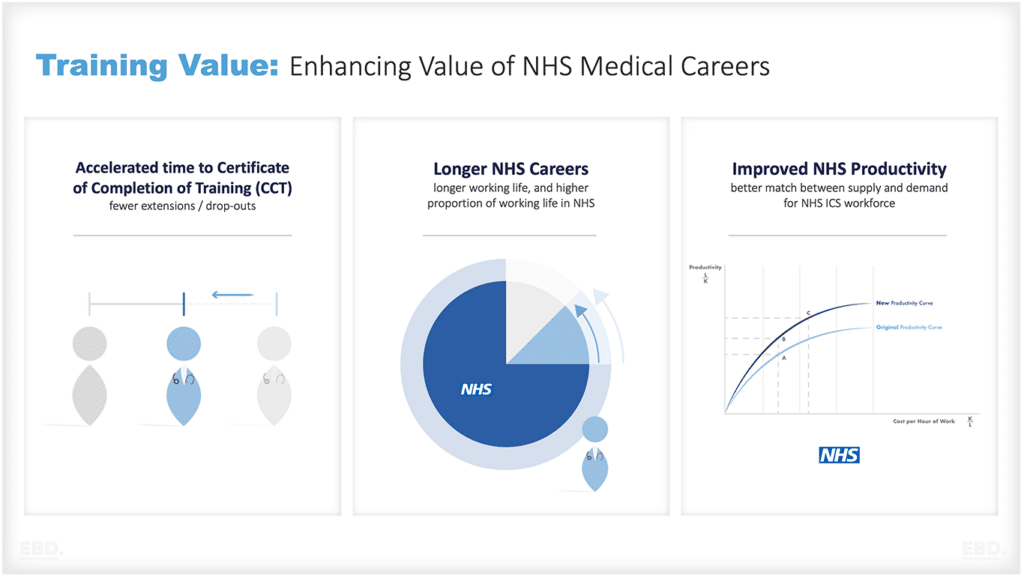
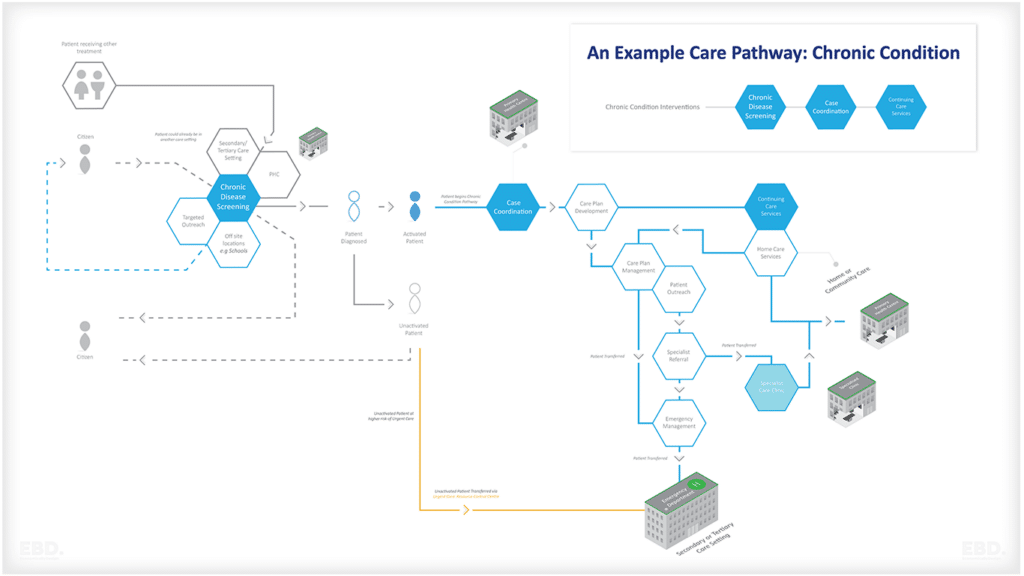
Pharmaceutical Value
The pharmaceutical industry is a significant contributor to the global economy and to health systems and health care across the world.
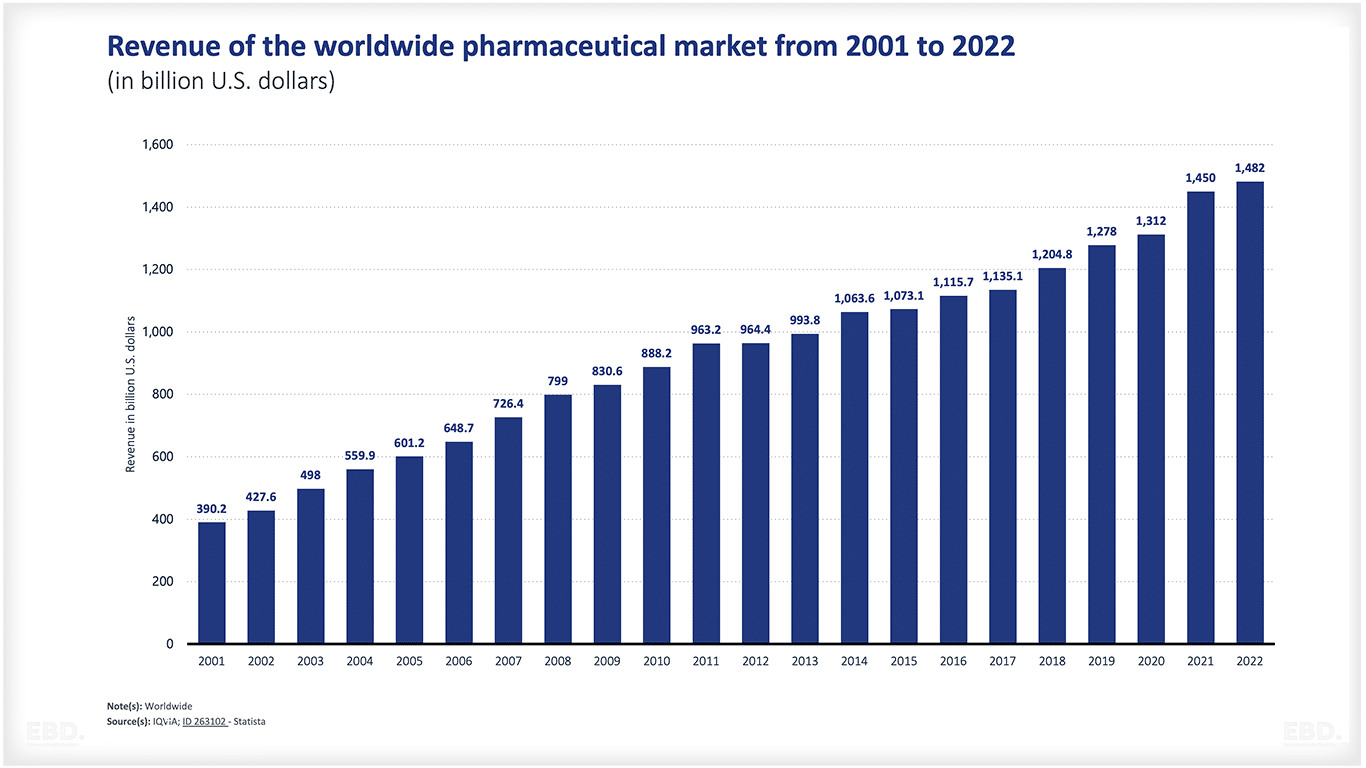
According to data from IQVIA, revenue in the global pharmaceuticals market is estimated to have reached US $1,482bn in 2022. Other estimates place the value even higher.
In this economics lens we take a look at pharmaceutical products, the pharmaceutical industry, the market for pharmaceuticals, and what drives pharmaceutical value.
What is a pharmaceutical product?
Pharmaceutical products are chemicals developed to provide medicines and drugs to control and treat disease. Records of the use of medicines date back some 30,000 years, but pharmaceutical science is a far more recent phenomenon. The development of the modern pharmaceutical industry is relatively recent having originated in the early 19th Century.
Pharmaceutical companies use complex terminology when describing products and here are some of the common terms.
Therapeutic classes
A therapeutic class is a group of pharmaceuticals that are used to treat similar medical conditions or illnesses. A therapeutic class may contain one drug, in which case it would be considered a single-entity drug product; or it may include several drugs, which is known as combination products. Some commonly prescribed therapeutic classes include antihypertensives, antidepressants and analgesics.
Novel Drugs
A novel drug is often defined as a new, innovative drug that is different to drugs that have been approved before. However, the term is also used to describe a drug that is available for patients with previously unmet public health needs. A recent example is the FDA approval of Daybue to treat Rett syndrome which is a neurodevelopmental disorder.
Drug Patents
A patent is a legal protection for inventors of new pharmaceutical products. Patent holders have exclusive rights to make, use and sell their inventions for 20 years from the date that the patent was filed. Once the patent is expired, other manufacturers can produce generic versions of the drug. 20 years is thought to provide enough time to recoup research and development costs and return a profit.
In practice, companies have protection for a more limited amount of time post-launch as it can take a long time for the product to be approved for use. Patents are not international so technically need to be applied for in the countries where the product is to be sold. There is some harmonisation of patents as part of the Trade Related Aspects of Intellectual Property Rights (TRIPS) administered by the World Trade Organisation.
Generic Drugs
A generic drug is a pharmaceutical product that is created to be the same as a brand-name drug. Generic drugs are usually much cheaper than the brand equivalent and are generally only different in flavour, colour, shape etc.
Generic drugs can be developed following the patent expiration of an existing brand product. However, some countries, that have not yet developed or recognised patents, allow in-country manufacture of generic drugs. A common example of a generic drug is paracetamol which is also marketed at a higher price as a branded product such as panadol.
Biological Drugs
Biological drug products, also known as biologics, are medically important substances produced with the help of living organisms or their components. Biological drugs are increasingly becoming popular due to their effectiveness in treating chronic diseases such as rheumatoid arthritis, psoriasis, Crohn’s disease and multiple sclerosis. Examples of biological drugs include bevacizumab (Avastin) for people with cancer and adalimumab (Humira) for people with arthritis.
Biosimilars
Owing to the complexity of biological drugs, generic versions are not always possible. As such there have been attempts to develop biosimilars which are similar in therapeutic effect to a reference biologic product. They may only be similar and not identical as they may contain small differences in terms of composition, molecular structure or impurities. However, these minor changes would not be clinically meaningful.
Orphan Drugs
Orphan drugs are those used to treat rare diseases or conditions and generally have very low sales volume compared to other medications. These drugs can be expensive to create and typically do not offer a good return on investment for pharmaceutical companies. However, there are incentives in many countries including market exclusivity and tax credits which can be awarded when a company develops an orphan drug.
Examples of orphan drugs include for multiple sclerosis and Kalydeco (ivacaftor) for cystic fibrosis and Alglucerase: A treatment for Gaucher disease, which causes pain and damage to tissue in the liver, spleen, lungs and bone marrow.
Prescription Drugs
Prescription drugs are medications that can only be obtained with a licenced clinician’s prescription. Examples of drugs that are usually only legally obtained with a prescription include antibiotics, such as penicillin, opioids, such as oxycontin and benzodiazepines, like valium.
Prescription requirements are usually in place where there is potential for misuse or abuse and there is a need to ensure that the drug is used safely and effectively by a medically qualified person. Prescriptions are also in place for drugs where there is a subsidy in place to cover the cost of the drug for the patient.
Over-The-Counter (OTC)
Over-the-counter, or nonprescription drugs, are medicines that do not need a prescription from a doctor and can be bought at stores. These medications are generally considered safe to use by the general public and typically treat common ailments such as pain, coughs and colds. Examples of over-the-counter medications include ibuprofen for pain, loratadine for allergies and guaifenesin for coughs.
Indications
A drug or medicine is usually approved for use for a particular medical condition, known as the ‘indication’. Some drugs can be used for multiple indications. For example, oncology (cancer) drugs are often approved for multiple indications. Sometimes drugs are prescribed “off label”, in other words, they are used to treat conditions other than the approved indication. Usually, this only happens if there is no alternative treatment available for the patient and it is considered to be of some therapeutic value.
Vaccines
Vaccines are a type of biological preparation usually made from weakened or killed forms of the organisms that cause particular diseases. Vaccines help to activate an individual’s immune system and prepare it to fight off future infections from the same virus. They are typically given as injections but may also be administered orally or through a nasal spray. Examples of successful vaccine development include the measles, mumps and rubella vaccine (MMR) and the hepatitis B vaccine.
The COVID-19 pandemic saw a huge global scientific effort to fast-track the development of safe and effective COVID-19 vaccines. The World Health Organisation encouraged countries to ensure that at least 70% of their populations were vaccinated. The development of vaccines was fast-tracked by staggering pre-clinical and clinical trials in parallel whereas these are normally done in sequence, consecutively. A really useful overview of how the development time was fast-tracked can be found on the European Medicines Agency website.
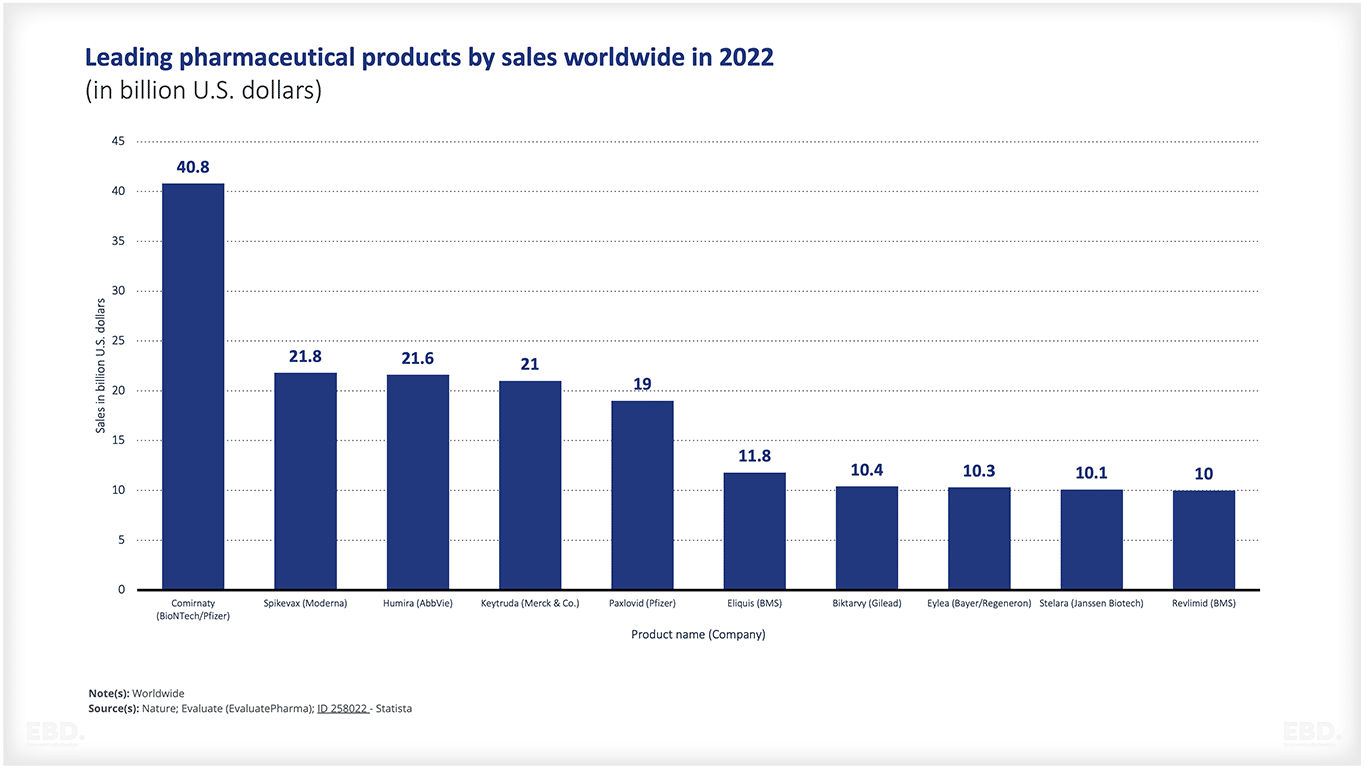
Top 10 pharmaceutical products worldwide
The figure below shows the projected top 10 pharmaceutical products based on lifetime sales. As can be seen, Comirnaty (2020) is already included in the list. Comirnaty is the name of the BioNTech Pfizer vaccine, one of the recently developed and approved vaccines design to prevent and mitigate the impact of Covid-19.
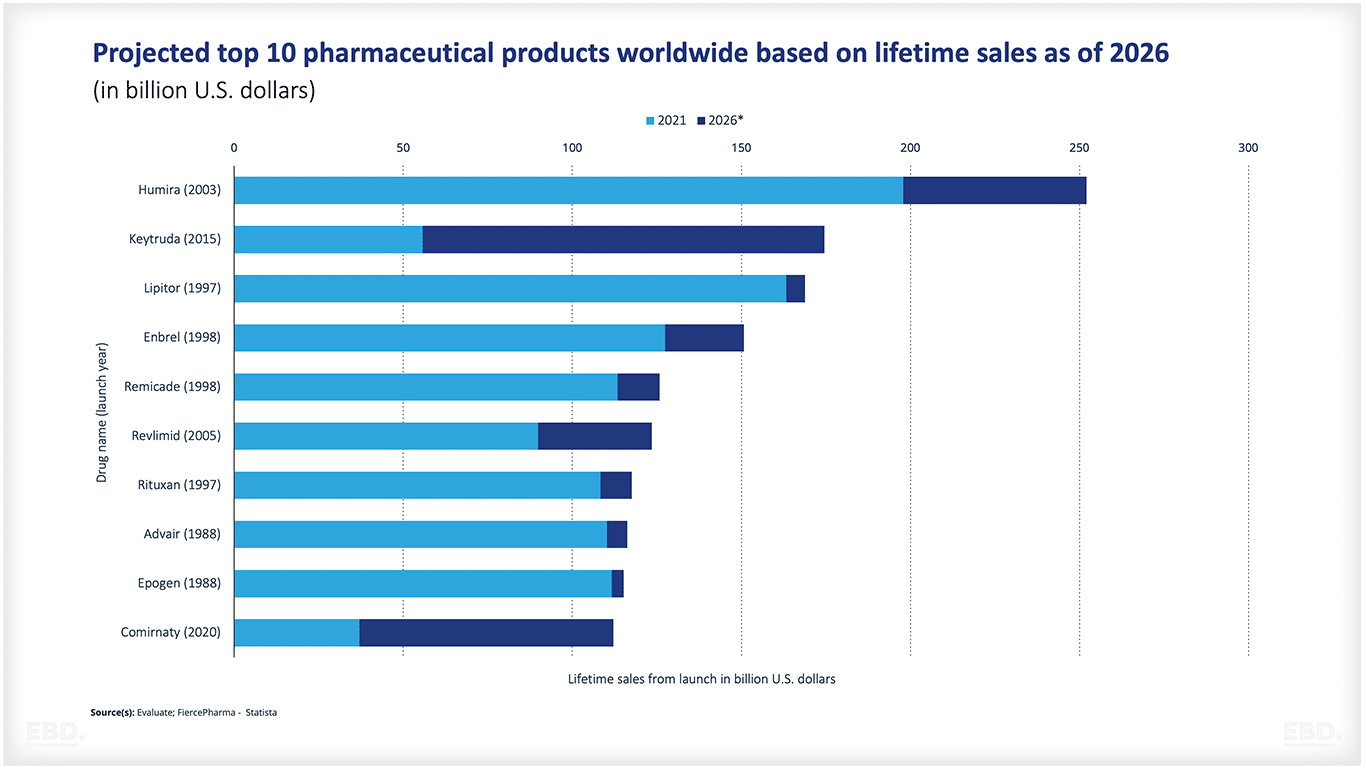
How big is the pharmaceutical industry?
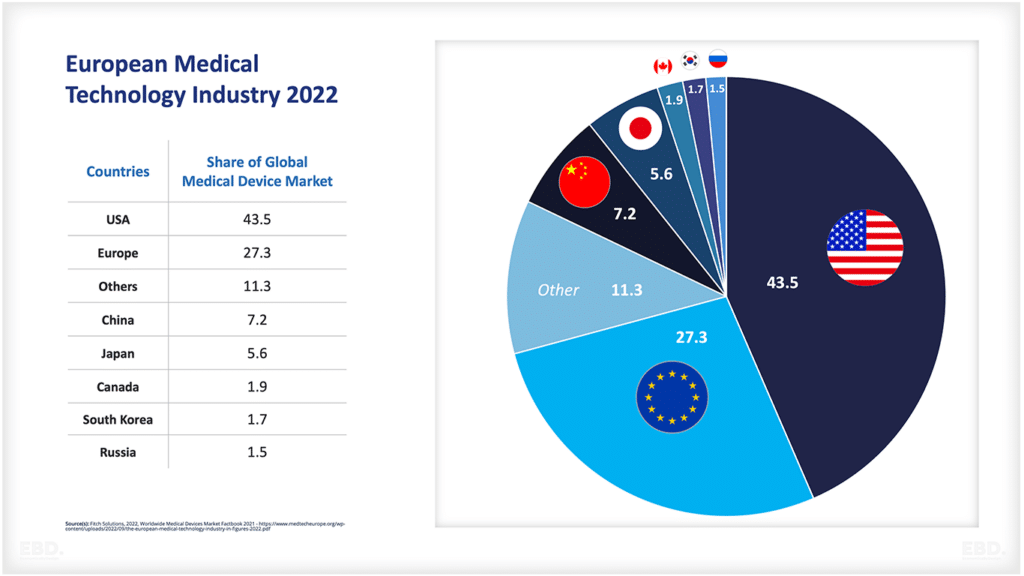
As mentioned above, revenue in the global pharmaceuticals market is estimated to have reached US$ 1,482 bn in 2022. The USA is by far and away the largest market over 4 times the size of the next largest market, namely China.
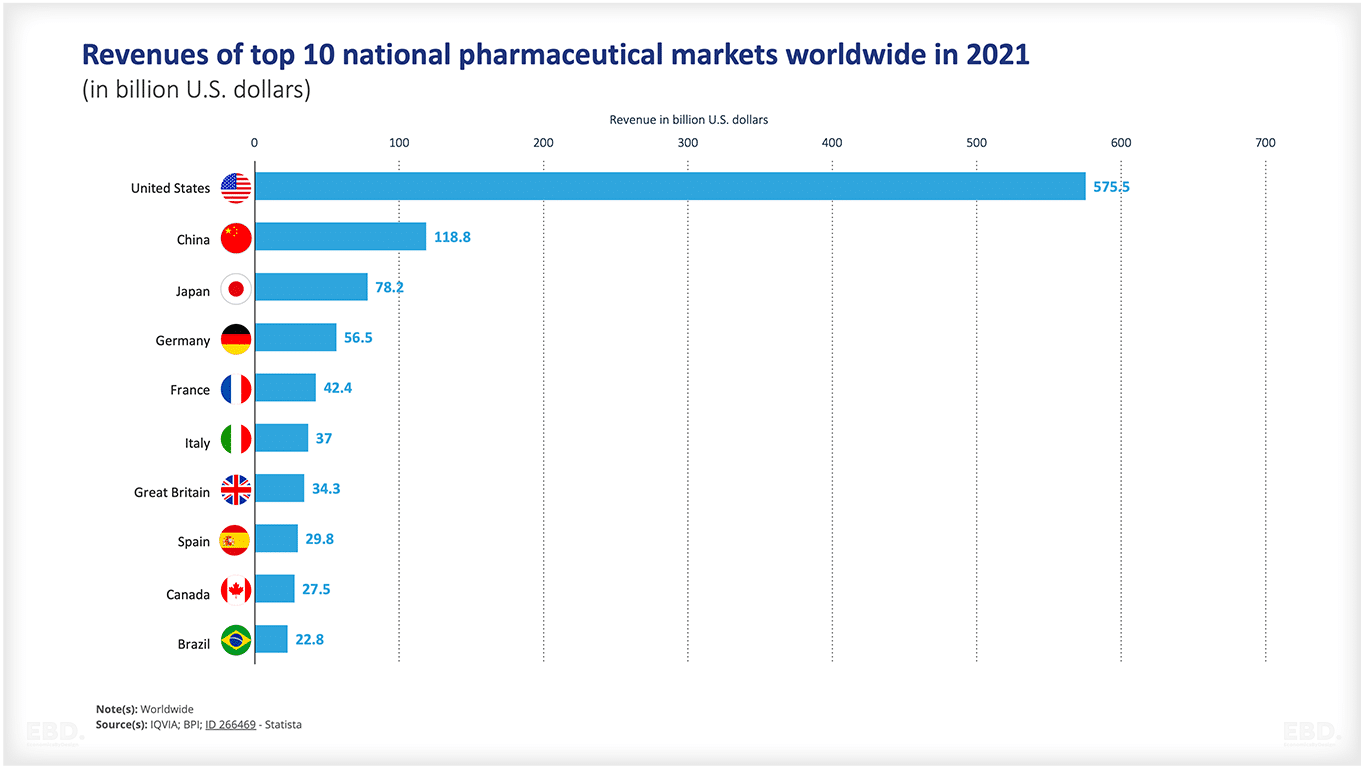
United States | 575.5 |
China | 118.8 |
Japan | 78.2 |
Germany | 56.5 |
France | 42.4 |
Italy | 37 |
Great Britain | 34.3 |
Spain | 29.8 |
Canada | 27.5 |
Brazil | 22.8 |
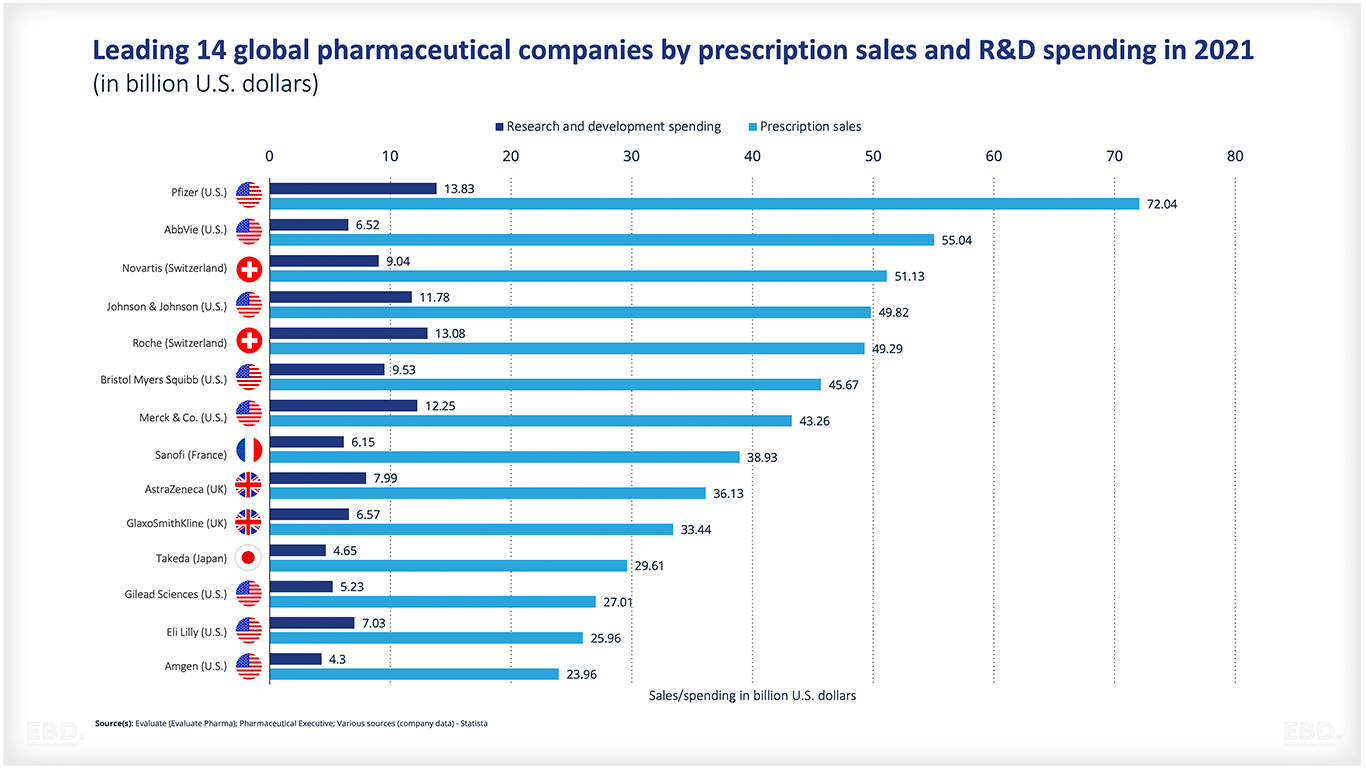
Which are the main pharmaceutical companies?
Examples of the leading global pharmaceutical companies include:
Some of these companies focus on particular therapeutic classes. Novo Nordisk for example is focused on diabetes. Others have broad portfolios such as J&J and Pfizer.
Research and development
All pharmaceutical companies invest heavily in research and development relative to prescription sales. According to the UNESCO Institute of Statistics , the world spent the equivalent of $2.47tn on research and development in 2022 Of this, $238bn relates to pharmaceutical research and development and this is expected to grow to $285bn by 2028. Further information on research and development can be found here.
What determines the price of a pharmaceutical product?
Economic theory suggests that the price of a pharmaceutical product is determined by the interaction between demand and supply and settles at a value which matches a customer’s willingness to buy and a producer’s willingness to sell.
Put simply, pharmaceutical companies will want to set prices across their portfolio of products to ensure costs are recovered, including the large costs of research and development, and that profits are maximised. Consideration will be given to the potential volume of sales across different countries and whether prices can vary for different geographies too. They will be mindful of the existence of alternative treatments, the development of generics (especially where a country has a relatively relaxed patent system), and the existence of price or tendering regulations that might exist in a given jurisdiction (3).
Those paying for drugs on behalf of health systems will want to ensure that they pay the lowest price possible whilst ensuring that patients have access to existing and new drugs. It is in their interest for pharmaceutical companies to succeed, but without encouraging individual companies to use their market power to generate “super profits”. Having approved a drug for use, an insurer or a government will be looking to achieve the lowest possible price for that drug, taking into account the benefits of the drug to the relevant population.
The NHS in England has an arms-length government agency whose role includes the assessment of the value of a new drug or medicine to the health system. The National Insitute for Health and Care Excellence (NICE) has developed world-influencing tools and methodologies for assessing the value of new treatments. Whilst it doesn’t set the price for a new drug, pharmaceutical companies seeking to launch a new drug in the UK (and other countries that use NICE recommendations as a benchmark of value) will take the value calculations undertaken by NICE into consideration in their pricing strategies.
Health Technology Assessment
The tools and methodologies used by NICE to assess the value of new treatments are an example of Health Technology Assessment (or HTA). Health Technology Assessment is increasingly used by countries across the world to assess the value of new treatments and in particular pharmaceuticals. The World Health Organisation is taking a leading role in building capacity for HTA and the adoption of consistent, systematic, multidisciplinary approaches.
HTA involves comparing the performance of a drug (or other treatment) with its alternatives. Performance issues will include an assessment of the effect of the drug on outcomes (the therapeutic effect), potential side effects or other impacts, how it impacts the quality of life, and how it is delivered. This will involve a review of the evidence from the clinical trials etc and other published research, and often will involve expert elicitation to interpret the results and appraise the evidence.
Many organisations that are responsible for paying for healthcare share information and are happy to accept evidence from others as part of the assessment process. A good example of this is the cooperation across the EU Member States. However, the appraisal itself is very dependent on the decision-making body and what is important in the local context.
Economic value such as used by NICE, involves a comparison of costs and benefits. Utility measures such as the Quality Adjusted Life Year (QALY) are used to measure the benefits to patients and the value assessment is done based on the cost per QALY. The value here is measured based on the impact on mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Sometimes the drug results in cost-savings – in other words, it is cheaper than the alternative (once health system cost consequences are taken into account) and delivers more benefit (positive QALY value). However, more often the new treatment adds to cost. Decision makers need to make a judgement about how high the threshold should be before a drug is considered too expensive. More details on the economic techniques and the QALY can be found here.
Decisions regarding whether a particular pharmaceutical should be included as part of the health benefits package and made available to patients will also include a wider set of stakeholders and include wider considerations about finance and budget and price. This phase is often termed Health Technology Appraisal (as opposed to assessment).
What are the five main challenges facing pharmaceutical companies?
There are many challenges facing pharmaceutical companies. Here are five main challenges faced right across the industry.
Variations in regulation across the world
Different countries have different product and price regulations and different processes for authorising drugs, assessing drug value and providing market access. It can be difficult to keep up to date and comply with all of them.
A comprehensive regulatory system incorporates oversight of pharmaceuticals from the laboratory to post-launch ensuring that the products are safe and effective and any safety issues or side effects are well understood so that risks of harm can be mitigated.
The World Health Organisation has developed a strategy for the development of consistent and harmonised regulatory requirements globally to protect the public whilst also providing an environment that enables access to products and innovation.
Increasing costs of research and development
As a result of the high and increasing costs associated with developing new drugs, companies have to carefully consider which drugs they invest in and how they cover abortive costs through the pricing of products that are ultimately launched. Only 7.8% of drugs move from pre-clinical to approval. That is a huge amount of resources devoted to products that do not make it to commercial launch.
There are innovations which might help reduce the costs of research and development. These include the use of digital health data (sometimes referred to as real-world data) to help focus research, reduce the costs of clinical trials, and provide important post-launch monitoring information. Digital tools are also of value enabling more efficient recruitment of participants for clinical trials and more cost-effective surveillance and monitoring through tools, devices and implants.
Governments and other relevant stakeholders will often subsidize or support research and development into new drugs, particularly where there is an unmet need or an urgent health emergency such as the COVID-19 pandemic that needs to be addressed.
Increasing competition
The industry is increasingly crowded with companies competing for market share, leading to new initiatives such as collaborations and mergers to stay ahead of the competition. Governments increasingly seek to ensure that there is competition in the pharmaceutical industry to mitigate against the risk that only a handful of companies will dominate the market as monopolies in key therapeutic classes.
This takes the form of control over mergers and other collaborations that would distort the market. The European Commission for example operates a strict competition policy in relation to pharmaceutical companies to prevent companies from obtaining too much market power.
Counterfeiting and price erosion
Counterfeiting is a growing problem that needs to be addressed as it affects not only the company’s bottom line but also public health. Estimates suggest that the global market in counterfeit medicines may be as large as US$432 bn (2). It is important not to confuse counterfeits and generics or biosimilars.
Generics and biosimilars are regulated and are safe and effective. Counterfeits are products which are falsely labelled and or have incorrect amounts of ingredients. Counterfeits can be found on the internet, in street markets and even in pharmacies and hospitals.
Skills shortages
The industry faces a shortage of skilled scientists with some reports suggesting a significant “talent gap” across the industry. Automation and technology will help to mitigate these risks but generally speaking a shortage of skilled workforce results in higher costs and higher costs will impact the price and availability of medicines.
What is the Economic Value of Pharmaceuticals
The economic value of pharmaceuticals is measured in terms of the benefit they bring to society. Pharmaceuticals help people live longer, healthier lives, reduce suffering from diseases and improve quality of life.
The pharmaceutical industry also contributes to the economy. Pharmaceutical companies are major employers and stimulate further employment across their supply chains.
Estimates suggest the global pharmaceutical industry directly contributed US$ 532 bn (1%) to the global Gross Domestic Product (GDP). Employing a workforce of over 5.5 million, and also stimulated the development of a further 45 million jobs across the supply chain, many in developing countries (4).
That said, the profitability of pharmaceutical companies is relatively high compared with equivalent-sized companies (5) even after allowing for research and development costs. There is an ongoing debate about whether the pharmaceutical industry is working as well as it should.
“Driven by profit rather than public health, the pharmaceutical sector is incentivised to set high prices and deliver short-term returns to shareholders, rather than focus on riskier, longer-term research which leads to critically needed therapeutic advances. The high prices of medicines are causing severe patient access problems worldwide, with damaging consequences for human health and wellbeing.” (6)
Useful References
(1) IQVIA January 18 2023. Revenue of the worldwide pharmaceutical market 2001-2022 (in billion U.S. dollars) In Statista Retrieved May 01, 2023, from //www.statista.com/statistics/263102/pharmaceutical-market-worldwide-revenue-since-2001
(2) Ofori-Parku SS. Fighting the global counterfeit medicines challenge: A consumer-facing communication strategy in the US is an imperative. J Glob Health. 2022 Apr 23;12:03018. doi: 10.7189/jogh.12.03018. PMCID: PMC9031510.
(3) Janssen Daalen JM, den Ambtman A, Van Houdenhoven M, et alDeterminants of drug prices: a systematic review of comparison studies BMJ Open 2021;11:e046917. doi: 10.1136/bmjopen-2020-046917
(4) Prof. Dr. Dennis Ostwald, Dr Marcus Cramer, Nora Albu, Jasmin Tesch: “The Global Economic Impact of the Pharmaceutical Industry” September 2020.
(5) Ledley FD, McCoy SS, Vaughan G, Cleary EG. Profitability of Large Pharmaceutical Companies Compared With Other Large Public Companies. JAMA. 2020 Mar 3;323(9):834-843. doi: 10.1001/jama.2020.0442. PMID: 32125401; PMCID: PMC7054843.
(6) UCL Institute for Innovation and Public Purpose. The people’s prescription: re-imagining health innovation to deliver public value. 2018. www.ucl.ac.uk/bartlett/public-purpose/sites/public-purpose/files/peoples_prescription_report_final_online.pdf.